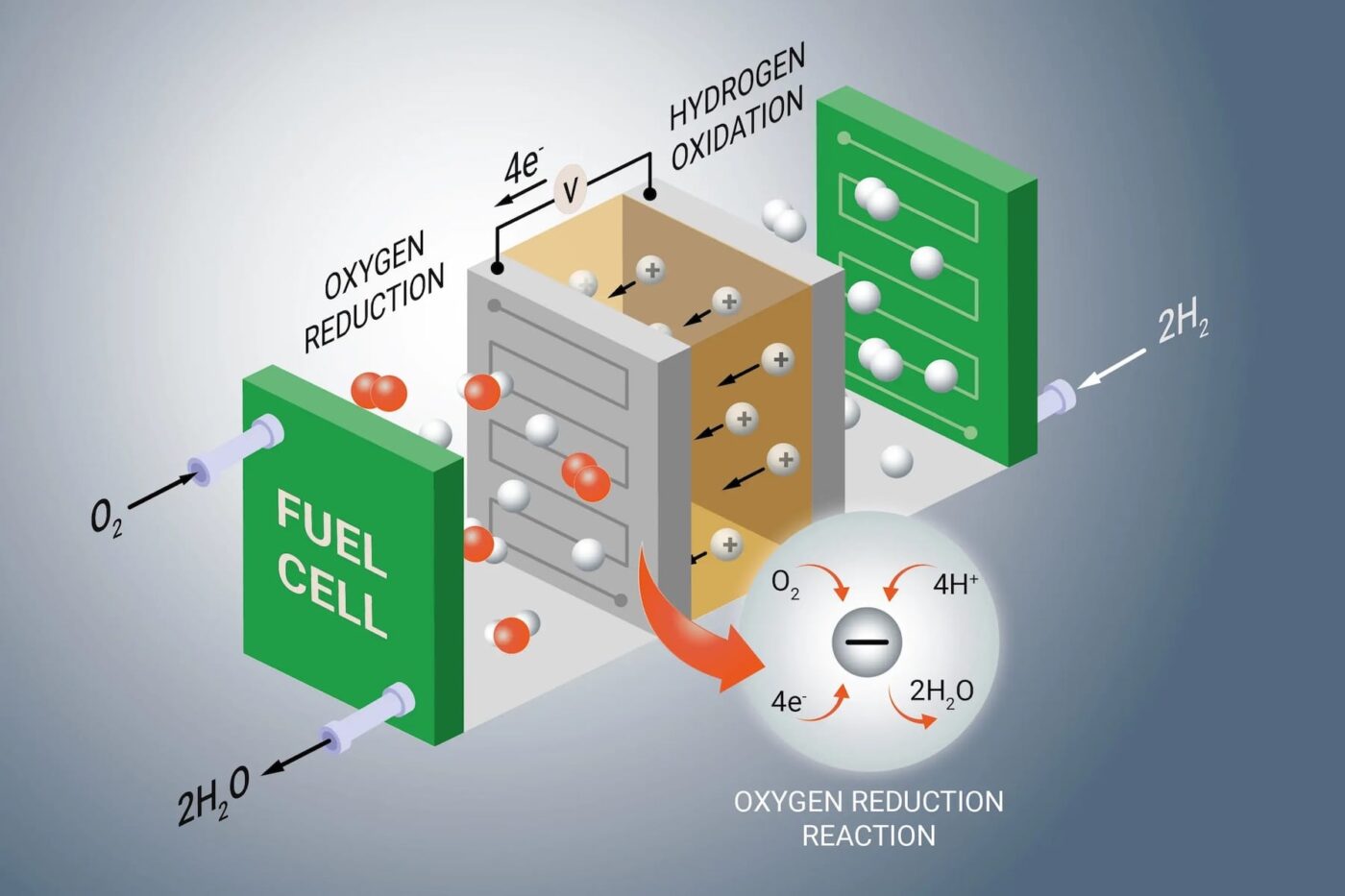
How fuel cell catalysts work
An important point to note upfront: unlike with internal combustion engines, catalysts in fuel cells are not used to clean exhaust gases but to generate energy. This is because, in a fuel cell, hydrogen is not burned as it is in an internal combustion engine. Instead, it is converted into electrical power. Catalysts are employed to accelerate this process.
The critical reactions at the anode (where hydrogen is split into protons and electrons) and the cathode (where oxygen, protons, and electrons react to form water) occur very slowly on their own. Catalysts speed up these reactions, making them technically viable. Without catalysts, virtually no electricity would be generated by the fuel cell.
However, the electrochemical processes in fuel cell catalysts remain relatively under-researched. This gap was addressed by the Fritz Haber Institute, which systematically investigated how the activity of four different fuel cell catalysts depends on voltage and pressure.
The findings revealed that catalyst activity cannot be attributed to a single rate-limiting reaction step (a bottleneck effect). Instead, it is influenced by various rate-determining steps, depending on the applied voltage.
Dr Sebastian Öner, head of the research team, explains: “For decades, researchers have frequently applied analyses and theories based on the assumption that there is a single rate-determining reaction step. Our work breaks with this tradition. We provide a kinetic framework for analysing operando spectroscopy and microscopy, which have been used for decades to study voltage-dependent structural and chemical changes. A central question is how overpotential- and pressure-dependent dynamic, microscopic properties influence the entire ensemble, ultimately defining the activation parameters. Our results provide new impetus for future research.”
In other words, while these new insights do not deliver a finished product, they fundamentally reshape our understanding of the core reaction in fuel cells. This creates a stronger foundation for materials research, simulations, and industrial development. The institute’s findings could, for example, help account for real-world operating conditions more effectively, refine catalyst development, reduce the platinum requirement for catalysts, and improve the robustness and durability of fuel cells.



0 Comments